many of the heavy metals are what to living things
Open access peer-reviewed chapter
Environmental Contamination past Heavy Metals
Submitted: October 6th, 2017 Reviewed: March 1st, 2018 Published: June 27th, 2018
DOI: 10.5772/intechopen.76082
From the Edited Volume
Heavy Metals
Edited by Hosam El-Din M. Saleh and Refaat F. Aglan
IntechOpen Downloads
15,841
Total Affiliate Downloads on intechopen.com
Altmetric score
Overall attention for this chapters
Abstruse
The surround and its compartments have been severely polluted by heavy metals. This has compromised the power of the environment to foster life and render its intrinsic values. Heavy metals are known to be naturally occurring compounds, but anthropogenic activities innovate them in large quantities in different environmental compartments. This leads to the environment'due south ability to foster life being reduced as human, animal, and constitute health go threatened. This occurs due to bioaccumulation in the food bondage equally a upshot of the nondegradable state of the heavy metals. Remediation of heavy metals requires special attention to protect soil quality, air quality, water quality, human health, animal health, and all spheres as a drove. Developed physical and chemical heavy metallic remediation technologies are enervating costs which are not viable, time-consuming, and release additional waste to the environment. This chapter summarises the bug related to heavy metallic pollution and diverse remediation technologies. A example report in Due south Africa mines were also used.
Keywords
- heavy metals
- surroundings
- contagion
- legal requirements
- pollution
*Address all correspondence to: masindivhahangwele@gmail.com
one. Surroundings
Surround can be referred to the surround within which humans exist. These are fabricated up of: the land, the h2o and the atmosphere of the earth; microorganisms, plant and animal life; any role or combination of the beginning 2 items on this listing and the interrelationships among and between them and the physical, chemic, aesthetic and cultural properties and conditions of the foregoing that influence human wellness and well-being. It is likewise characterised past a number of spheres that influence its behaviour and intrinsic value. The most important sphere of the environment is the biosphere because it harbours the living organisms. This is the sphere where you find living organisms (plants and animals) interacting with each and their nonliving environment (soil, air and water). In the late centuries, industrialisation and globalisation take impaired pristine environments and their power to foster life. This has introduced components that compromise the holistic functioning of the environment and its intrinsic values [1].
1.1. Environmental contamination
An environment can be polluted or contaminated. Pollution differs from contamination; still, contaminants tin be pollutants, and pose detrimental impact on the environment. From literature, pollution is divers as the introduction past man, directly or indirectly, of substances or energy into the environment resulting in such deleterious effects as impairment to living resource, hazards to human health, hindrance to environmental activities and harm of quality for use of the environment and reduction of amenities. Contamination on the other hand is the presence of elevated concentrations of substances in the environs to a higher place the natural background level for the area and for the organism. Ecology pollution tin can be referred to undesirable and unwanted alter in concrete, chemical and biological characteristics of air, water and soil which is harmful for living organisms—both animate being and plants. Pollution tin can accept the course of chemical substances or energy, such as racket, heat or light [2].
Pollutants, the elements of pollution, can either be foreign substances/energies or naturally occurring contaminants.
ane.1.1. Types of pollutants
Environmental pollutants continue to be a earth concern and one of the great challenges faced by the global society. Pollutants can be naturally occurring compounds or foreign matter which when in contact with the environment cause agin changes. In that location are different types of pollutants, namely inorganic, organic and biological. Irrespective of pollutants falling under unlike categories, they all receive considerable attending due to the impacts they introduce to the environment. The human relationship between environmental pollution and globe population has become an inarguable directly proportional human relationship every bit it can be seen that the amount of potentially toxic substances released into the environment is increasing with the alarming growth in global population. This consequence has led to pollution beingness a significant problem facing the surroundings.
1.1.1.1. Inorganic pollutants
Industrial, agricultural and domestic wastes contribute to ecology pollution, which cause agin impairment to human and animal health. From such sources, inorganic pollutants are released. Inorganic pollutants are unremarkably substances of mineral origin, with metals, salts and minerals being examples [2]. Studies take reported inorganic pollutants every bit textile found naturally just have been contradistinct by human production to increase their number in the surround. Inorganic substances enter the environment through different anthropogenic activities such as mine drainage, smelting, metallurgical and chemical processes, too as natural processes. These pollutants are toxic due to the accumulation in the food chains [3].
1.1.one.2. Organic pollutants
Organic pollution can be briefly defined equally biodegradable contaminants in an environment. These sources of pollution are naturally constitute and caused by the environment, simply anthropogenic action has as well been contributing to their intensive production to meet the human needs. Some of the common organic pollutants which have been noted to be of special business are human waste, food waste, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), polycyclic aromatic hydrocarbons (PAHs), pesticides, petroleum and organochlorine pesticides (OCPs) [4].
Organic pollutants have gained attention as they have go a major problem in the environs. Backdrop of organic pollutants, amongst others, such as high lipid solubility, stability, lipophilicity and hydrophobicity take recently made organic pollutants termed persistent. These backdrop requite organic pollutants the ability to hands bioaccumulate in the different spheres of the environment, thus causing toxicological effects [five, 6].
1.1.one.iii. Biological pollutants
Biological pollutants are described as pollutants which be as a result of humanity's actions and touch on the quality of aquatic and terrestrial environment. This type of pollutants include bacteria, viruses, moulds, mildew, animal dander and cat saliva, business firm dust, mites, cockroaches and pollen. Studies have documented different sources of these pollutants, including pollens originating from plants; viruses transmitted by people and animals; bacteria carried past people, animals, and soil and found debris [7].
Advertisement
two. Heavy metals
Although in that location is no specific definition of a heavy metallic, literature has defined it as a naturally occurring element having a high diminutive weight and high density which is five times greater than that of water [viii]. Among all the pollutants, heavy metals take received a paramount attending to environmental chemists due to their toxic nature. Heavy metals are normally nowadays in trace amounts in natural waters but many of them are toxic even at very low concentrations [ix]. Metals such equally arsenic, lead, cadmium, nickel, mercury, chromium, cobalt, zinc and selenium are highly toxic even in pocket-sized quantity. Increasing quantity of heavy metals in our resource is currently an expanse of greater concern, especially since a large number of industries are discharging their metal containing effluents into fresh water without whatever adequate treatment [3].
Heavy metals go toxic when they are non metabolised by the trunk and accumulate in the soft tissues. They may enter the man body through food, h2o, air or absorption through the pare when they come in contact with humans in agronomics, manufacturing, pharmaceutical, industrial or residential settings. Industrial exposure accounts for a common road of exposure for adults. Ingestion is the near common route of exposure in children. Natural and human activities are contaminating the environment and its resource, they are discharging more than what the environment can handle [ix, x] (Figure ane).
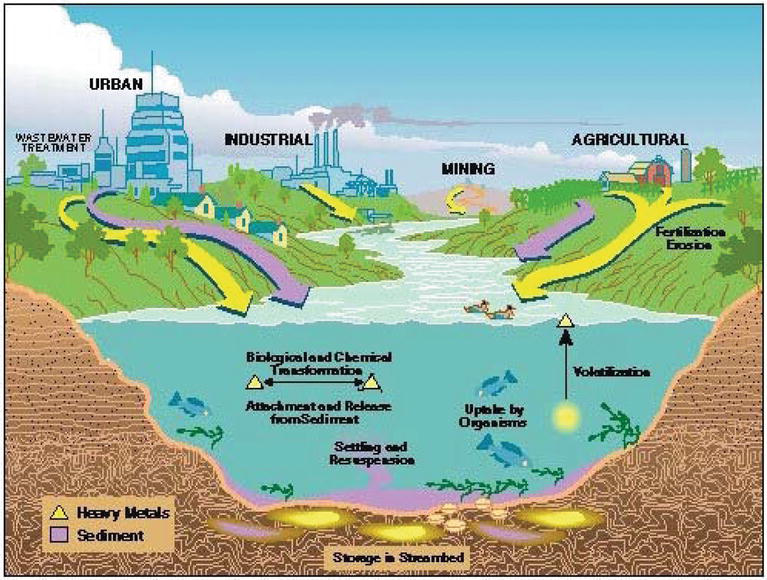
Effigy 1.
Sources and sinks of heavy metals [
ii.1. Sources of heavy metals
Heavy metals tin emanate from both natural and anthropogenic processes and cease upwards in different environmental compartments (soil, water, air and their interface) (Figure 2).
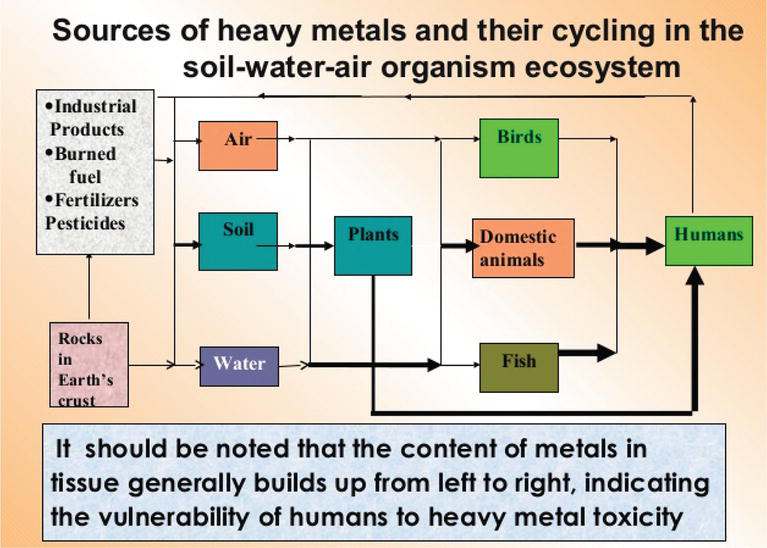
Figure 2.
Sources of heavy metals and their wheel in the environment [
2.1.1. Natural processes
Many studies accept documented different natural sources of heavy metals. Nether different and certain ecology conditions, natural emissions of heavy metals occur. Such emissions include volcanic eruptions, sea-common salt sprays, forest fires, rock weathering, biogenic sources and wind-borne soil particles. Natural weathering processes can lead to the release of metals from their endemic spheres to different environment compartments. Heavy metals can exist establish in the grade of hydroxides, oxides, sulphides, sulphates, phosphates, silicates and organic compounds. The most common heavy metals are lead (Lead), nickel (Ni), chromium (Cr), cadmium (Cd), arsenic (Every bit), mercury (Hg), zinc (Zn) and copper (Cu). Although the same heavy metals can be institute in traces, they still crusade serious health problems to human and other mammals [9].
2.1.two. Anthropogenic processes
Industries, agriculture, wastewater, mining and metallurgical processes, and runoffs also lead to the release of pollutants to different environmental compartments. Anthropogenic processes of heavy metals take been noted to go beyond the natural fluxes for some metals. Metals naturally emitted in wind-blown dusts are mostly from industrial areas. Some important anthropogenic sources which significantly contribute to the heavy metal contagion in the surround include automobile exhaust which releases pb; smelting which releases arsenic, copper and zinc; insecticides which release arsenic and burning of fossil fuels which release nickel, vanadium, mercury, selenium and tin. Human activities take been establish to contribute more to ecology pollution due to the everyday manufacturing of appurtenances to run into the demands of the large population [10].
2.2. Environmental impacts of heavy metals
The presence of heavy metals in the environment leads to a number of adverse impacts. Such impacts affect all spheres of the environment, that is, hydrosphere, lithosphere, biosphere and atmosphere. Until the impacts are dealt with, health and mortality problems suspension out, equally well every bit the disturbance of food chains. Figure iii summarises the wellness impacts of heavy metals.
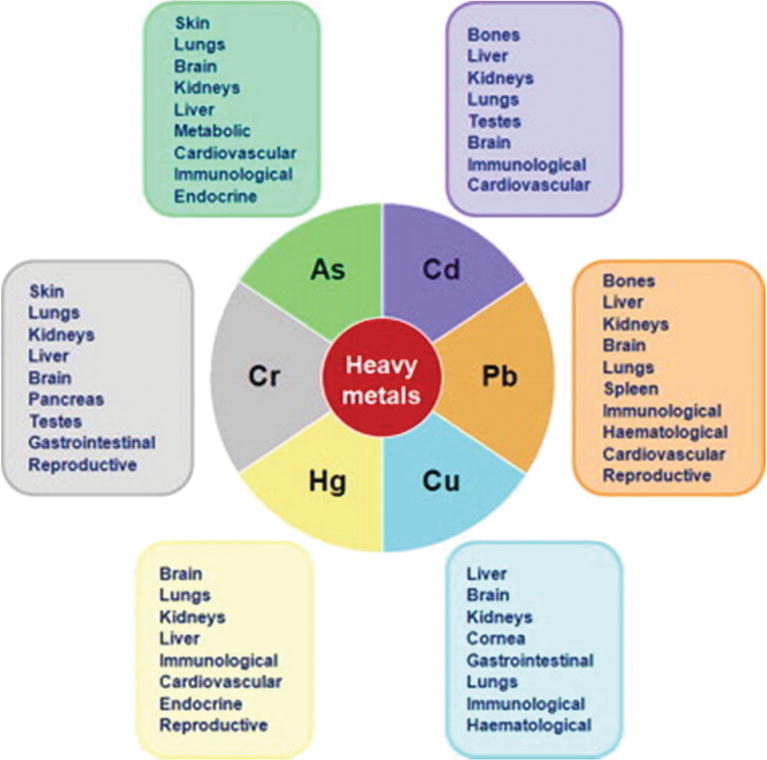
Figure iii.
Impacts of heavy metals on the environment [
2.three. Effect of heavy metals contamination
Heavy metals contamination is condign a serious result of concern around the earth as it has gained momentum due to the increase in the use and processing of heavy metals during various activities to meet the needs of the rapidly growing population. Soil, water and air are the major environmental compartments which are affected by heavy metals pollution.
two.iii.1. Upshot on soil
Emissions from activities and sources such equally industrial activities, mine tailings, disposal of loftier metal wastes, leaded gasoline and paints, country application of fertilisers, animate being manures, sewage sludge, pesticides, wastewater irrigation, coal combustion residues and spillage of petrochemicals atomic number 82 to soil contagion by heavy metals. Soils take been noted to be the major sinks for heavy metals released into the environment by same anthropogenic activities. Most heavy metals do not undergo microbial or chemic deposition considering they are nondegradable, and consequently their total concentrations last for a long time subsequently being released to the surroundings [5, 14].
The presence of heavy metals in soils is a serious effect due to its residence in food bondage, thus destroying the entire ecosystem. As much as organic pollutants can exist biodegradable, their biodegradation rate, however, is decreased by the presence of heavy metals in the environs, and this in plow doubles the environmental pollution, that is, organic pollutants and heavy metals thus present. There are various means through which heavy metals present risks to humans, animals, plants and ecosystems as a whole. Such means include direct ingestion, absorption by plants, food chains, consumption of contaminated water and amending of soil pH, porosity, colour and its natural chemistry which in turn impact on the soil quality [xv].
ii.3.2. Effects on water
Although in that location are many sources of water contamination, industrialisation and urbanisation are two of the culprits for the increased level of heavy metallic water contagion. Heavy metals are transported by runoff from industries, municipalities and urban areas. Most of these metals end up accumulating in the soil and sediments of water bodies [xv].
Heavy metals tin can be establish in traces in water sources and still exist very toxic and impose serious health bug to humans and other ecosystems. This is considering the toxicity level of a metallic depends on factors such as the organisms which are exposed to information technology, its nature, its biological part and the period at which the organisms are exposed to the metallic. Nutrient bondage and food webs symbolise the relationships amongst organisms. Therefore, the contagion of h2o past heavy metals really affects all organisms. Humans, an example of organisms feeding at the highest level, are more prone to serious wellness problems because the concentrations of heavy metals increase in the food chain [16].
2.3.three. Effects on air
Industrialisation and urbanisation, due to rapid earth population growth, have recently made air pollution equally a major environmental problem effectually the world. The air pollution was reported to have been accelerated by dust and particulate matters (PMs) peculiarly fine particles such equally PM2.v and PM10 which are released through natural and anthropogenic processes. Natural processes which release particulate matters into air include dust storms, soil erosion, volcanic eruptions and stone weathering, while anthropogenic activities are more industrial and transportation related [17].
Particulate matters are of import and require special attention as they can atomic number 82 to serious health problems such as skin and optics irritation, respiratory infections, premature bloodshed and cardiovascular diseases. These pollutants also crusade deterioration of infrastructure, corrosion, formation of acid rain, eutrophication and haze [9]. Amid others, heavy metals such as group 1 metals (Cu, Cd, Pb), group 2 metals (Cr, Mn, Ni, 5 and Zn) and grouping 3 metals (Na, Yard, Ca, Ti, Al, Mg, Fe) originate from industrial areas, traffic and natural sources, respectively [17, 18].
two.four. Mechanisms of remediating heavy metals
Treatment processes for acid mine water typically generate high-density sludge that is heterogeneous due to variety of metals, metalloids and anionic components, and this makes it hard to dispose the sludge [19]. Contempo researches accept therefore focused on the recovery of chemic species from acrid mine drainage (AMD) and secondary sludge. This is aimed at recovering valuable resources and as well enabling easier and safer disposal of the treated sludge, hence reducing their ecology footprints. Disposal of metal ladened waste to landfills and waste product retentiveness ponds/heaps lead to secondary pollution of surface and subsurface water resources. It may also pb to soil contamination, hence affecting their productivity [19].
In order to protect the homo health, plants, animals, soil and all the compartments of the environment, proper and conscientious attending should be given to remediation technologies of heavy metals. Most physical and chemical heavy metal remediation technologies require handling of large amounts of sludge, destroy surrounding ecosystems and are very expensive [19] (Figure 4).
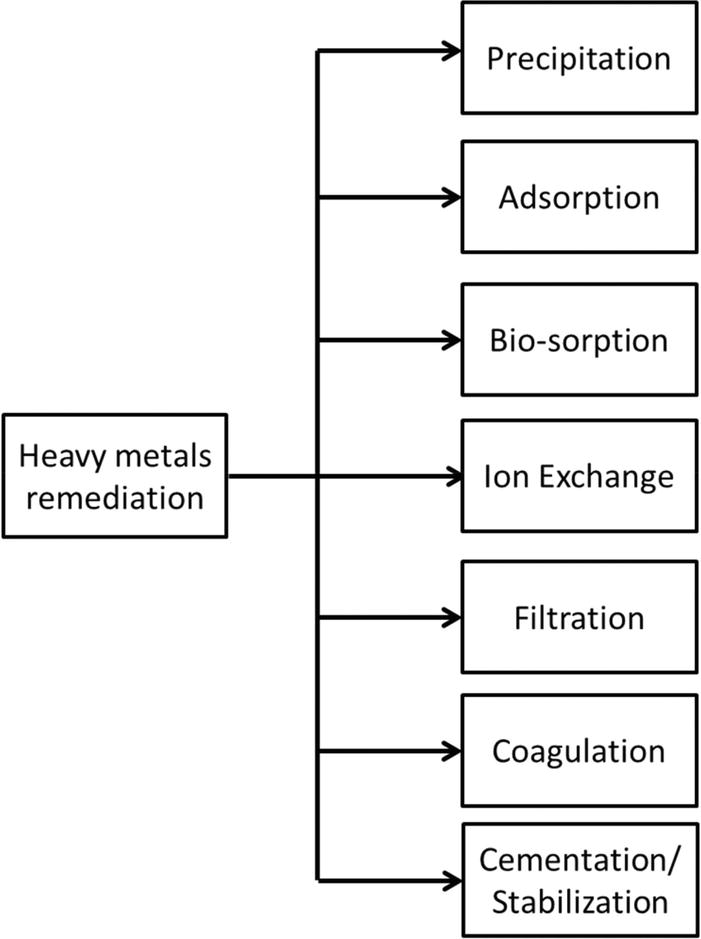
Effigy 4.
Mechanisms for the removal of heavy metals [
2.4.ane. Precipitation
A diverseness of alkaline chemical reagents have been used over the years for neutralisation of acid mine drainage (AMD) in order to increase the pH and consequently precipitate and recover the metals. The nearly mutual alkaline reagents used for sequential recovery of minerals resources from AMD are limestone (CaCO3), caustic soda (NaOH), soda ash (Na2CO3), quicklime (CaO), slaked lime (Ca(OH)2) and magnesium hydroxide (Mg(OH)two) [21]. Some processes take recovered metals at varying pH regimes (Table 1) and synthesised commercially valuable materials such every bit pigments and magnetite [22]. Some minerals are recovered and sold to metallurgical industries, hence off-setting the treatment costs [19].
| Metal ion | pH | Metal ion | pH | Metal ion | pH |
|---|---|---|---|---|---|
| Althree+ | iv.1 | Hg2+ | 7.iii | Cdtwo+ | 6.vii |
| Fe3+ | three.5 | Na+ | vi.vii | Fe2+ | five.five |
| Mn2+ | eight.five | Pb2+ | 6.0 | Cu2+ | 5.three |
| Crthree+ | 5.three | Zn2+ | 7.0 |
Table 1.
pH values at which metals in AMD precipitate [21].
2.4.two. Adsorption
Adsorption occurs when an adsorbate adheres to the surface of an adsorbent. Due to reversibility and desorption capabilities, adsorption is regarded the most effective and economically viable option for the removal of metals from aqueous solution. Although efficient, adsorption is not effective with very concentrated solution as the adsorbent hands gets saturated with the adsorbate. Information technology is but feasible for very dilute solutions, is labour intensive considering it requires frequent regeneration and information technology is not selective in terms of metal attenuation [21]. Adsorption is therefore not applied in a large scale of metal remediation.
2.iv.3. Ion exchange
Ion exchange is the exchange of ions betwixt two or more electrolyte solutions. Information technology tin also refer to exchange of ions on a solid substrate to soil solution. High cation exchange capacity clay and resins are ordinarily used for the uptake of metals from aqueous solutions. However, this method requires high labour and is limited to certain concentration of metals in the solution. This organization also operates under specific temperature and pH. Natural and synthetic clays, zeolites and synthetic resins take been used for removal and attenuation of metals from wastewater [19, 23].
ii.4.4. Biosorption
Biosorption refers to the removal of pollutants from water systems using biological materials, and it entails the assimilation, adsorption, ion substitution, surface complexation and precipitation. Biosorbents have an advantage of accessibility, efficiency and capacity. This process is readily and hands available. Regeneration is easy, hence making it very favourable. However, when the concentration of the feed solution is very high, the process hands reaches a breakthrough, thus limiting farther pollutant removal [24].
ii.4.5. Membrane technologies
The use of membrane technologies for the recovery of acid mine drainage is very effective for water that has high concentration of pollutants. Information technology uses the concentration gradients phenomenon or the reverse which is reverse osmosis. In that location are different types of membranes that are used for mine water treatment including: ultrafiltration, nano-filtration, contrary osmosis, microfiltration and particle filtration [19, 25, 26].
Advertizement
3. Example study of South Africa acid mine drainage
South Africa is well endowed past mineral reserves and this has triggered its immense dependence on mineral resources for gross domestic product and economy. However, the legacy of coal and gold mining has left in its wake serious environmental bug. The major problem is acid mine drainage. Acid mine drainage (AMD) is formed from the hydro-geochemical weathering of sulphide-bearing rocks (pyrite, arsenopyrite and marcasite) in contact with h2o and oxygen [23, 27]. This reaction is also catalysed past iron (Fe) and sulphur-oxidising microorganisms [28, 29]. In a nutshell, the germination of AMD can exist summarised as follows [19, 23, 30, 31]:
E1
The oxidation of sulphide to sulphate solubilises the ferrous iron (Iron(Ii)), which is subsequently oxidised to ferric atomic number 26 (Iron(Three)):
E2
Either these reactions can occur spontaneously or can exist catalysed by microorganisms (sulphur- and fe-oxidising leaner) that derive energy from the oxidation reaction [26]. The ferric cations produced tin can also oxidise additional pyrite into ferrous ions:
E3
The internet outcome of these reactions is to produce H+ and maintain the solubility of the ferric iron [32]. Because of the high acidity and elevated concentration of toxic and hazardous metals, AMD has been a prime issue of environmental business organisation that has globally raised public business [33].
The belch of metalliferous drainage from mining activities has rendered the environs unfit to foster life [22]. Businesslike approaches need to be developed to counter for this mining legacy that is perpetually degrading the surroundings and its precious resources [21]. Researches and piloted studies take indicated that active and passive approaches can be successfully adopted to treat acid mine drainage and remove potentially toxic chemical species [23, 31]. The presence of Al, Fe, Mn and sulphates is a prime concern in addition to the trace of Cu, Ni, Atomic number 82 and Zn [29]. Metalloids of Equally and earth alkali metal (Ca and Mg) are besides nowadays in significant levels [33]. Several studies have shown the feasibility of treating acid mine drainage to acceptable levels as prescribed by dissimilar water quality guidelines, but the resultant sludge has been an issue of public concern due to its heterogeneous and complex nature loaded with metal species [23, 34].
Based on that evidence, research studies have been firmly embedded on the recovery of valuable minerals from AMD [19, 23]. In that location are several mechanisms used for the recovery of chemical components from AMD including: precipitation [35], adsorption [36], biosorption [24], ion exchange [xix, 25, 26], desalination [37] and membrane filtration [38, 39]. Out of those techniques, atmospheric precipitation has been the promising technology due to the ability to handle big volumes of water with very trivial dosage [35]. Adsorption and ion exchange have a challenge of poor efficiency at elevated concentrations and quick rate of saturation. Membrane technologies accept the problem of generating brine that creates some other environmental liability. Desalination has a problem of producing salts that has impurities, hence making them unsuitable for utilisation. Freeze desalination has been the promising technology, only information technology has never been tried in a large calibration [nineteen, 23, 34].
3.i. Impacts of heavy metals in Due south Africa
South Africa'due south geology is rich in coal and mineral reserves which incorporate key metals such as gilt, platinum and copper. The meaning volume of mineral and coal reserves has fabricated mining serve as a backbone in the development and growth of the state's economy. This is evident from the massive number of mines establish effectually the state. However, mining has been noted to cause inimical impacts to the human health, organisms and surround as a whole, with water resources being the nigh common victim of the pollution [40].
The mining of coal and gold for multilateral uses exposes pyrite to oxidising agents. Atomic number 26 hydroxide and sulphuric acrid are toxic chemical species to living organisms when introduced into water resources (both surface and underground). This deteriorates the natural form of the water bodies and its ability to foster life. Acid mine drainage has very low pH of nigh <i.4 to >three [41, 42]; high TDS, EC and other metals in toxic concentration. Previous studies documented the following concentrations in AMD: < 75 ppm to >47,800 acidity; <3560 to >41,700 SO42- ppm; <460 to >12,270 ppm full Fe; <17,400 to 37,700 μg/L Zn; <270 to >13,000 μg/L Cu; <520 to >1500 μg/L Co; <75 to >360 μg/L Ni; <8 to >30 μg/L Pb and 6 to 30 μg/L Cd [41, 42, 43, 44].
However, the above-mentioned concentrations depend on the pH of the AMD—concentrations decrease when pH increases. When exposed to such conditions, mortality and diseases are nigh likely to occur in organisms, as well equally other health [45]. In addition, AMD destroys ecosystems of organisms and also negatively impacts on the economic system of the country. Heavy metals in active and abandoned mines in South Africa have impacted both surface and underground h2o.
3.2. Legal requirements of h2o quality
The National Ecology Management Act (NEMA) 108 of 1998, stipulates that everyone has the right to live in an environment which is safe and unlikely to pose any deleterious effects to their health. The legislative requirements for industrial effluents are primarily governed by the Section of H2o Diplomacy DWS H2o Quality Guidelines [46]. This purpose requires that any person who uses water for industrial purposes shall purify or otherwise treat such water in accordance with requirements of DWA [41, 46, 47, 48]. The relevant criteria for belch of acidic and sulphate-rich water are given in Table ii.
| Parameter | Gilt AMD* | Coal AMD** | Neutral drainage† | DWS industrial | DWS irrigation |
|---|---|---|---|---|---|
| pH | 2.3 | 2.5 | 6.5 | 5.0–10.0 | 6.five–8.4 |
| EC | 22,713 | 13,980 | 500 | 0–250 | >540 |
| Na | 248.4 | 70.5 | twenty.1 | — | 430–460 |
| 1000 | 21.6 | 34.two | 29.one | — | — |
| Mg | 2.iii | 398.nine | 861.8 | — | — |
| Ca | 710.8 | 598.7 | 537.5 | — | — |
| Al | 134.4 | 473.ix | 0.01 | — | 5.0–twenty |
| Iron | 1243 | 8158.2 | 0.07 | 0.0–ten | five.0–20 |
| Mn | 91.5 | 88.ii | 25.0 | 0.0–10.0 | 0.02–10.0 |
| Cu | 7.eight | — | — | — | 0.2–5.0 |
| Zn | 7.nine | viii.36 | 0.sixteen | — | 1.0–v.0 |
| Pb | 6.three | — | — | — | 0.2–two.0 |
| Co | 41.iii | 1.89 | 0.29 | — | 0.05–5.0 |
| Ni | 16.6 | 2.97 | 0.21 | — | 0.2–2.0 |
| SOiv 2− | 4635 | 42,862 | 4603 | 0–500 | — |
Table 2.
The relevant criteria for belch of acidic and sulphate-rich h2o as compared to DWS h2o quality guidelines.
Gilt mining AMD [44].
Coalmining AMD.
Neutral drainage water [40, 42, 45, 47, 48, 49, fifty, 51].
As shown in Table 2, mine effluents in S Africa are dominated by dissolved Fe, Al, Mn, Ca, Na, Mg and traces of Cu, Co, Zn, Pb and Ni. These concentrations are far above the legal requirements.
Advert
4. Deleterious furnishings of acid mine drainage on terrestrial and aquatic ecosystems
The introduction of effluents from mining activities into receiving streams can severely impact aquatic ecosystems through habitat destruction and impairment of water quality. This will eventually lead to reduction in biodiversity of a given aquatic ecosystem and its ability to sustain life. The severity and extent of damage depends on a diverseness of factors including the frequency of influx, volume and chemistry of the drainage and the buffering capacity of the receiving stream [22, 52, 53, 54, 55, 56, 57, 58].
4.one. Acerbity
When metals in AMD are hydrolysed, they lower the pH of the h2o making it unsuitable for aquatic organisms to thrive [52]. AMD is highly acidic (pH ii–4), and this promotes the dissolution of toxic metals [44]. Those toxic species exert chancy effects on terrestrial and aquatic organisms [23]. As well, if the water is highly acidic, only acidophile microorganisms will thrive on such water with the residual of aquatic organisms migrating to other regions which are conducive to their survival. Many streams contaminated with AMD are largely devoid of life for a long manner downstream. To some aquatic organisms, if the pH range falls below the tolerance range, probability of death is very high due to respiratory and osmoregulation failure. Acidic conditions are dominated by H+ which is adsorbed and pumps out Na from the body which is important in regulating body fluids [23, 52, 53, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65].
4.two. Toxic chemical species
Exposure of aquatic and terrestrial organisms to potentially toxic metals and metalloids can have devastating impacts to living organisms [44, 66, 67]. Toxic chemical species nowadays in AMD take been reported to be toxic to aquatic and terrestrial organisms. They are associated with numerous diseases including cancers. Some of these chemical species may accrue and be biomagnified in living organisms, hence threatening the life of higher trophic organisms such as birds [68]. Lead causes blood disorders, kidney impairment, miscarriages and reproductive disorders and is linked to various cancers. The exposure of living organisms to toxic chemic species in AMD can also atomic number 82 to nausea, diarrhoea, liver and kidney harm, dermatitis, internal haemorrhage and respiratory issues. Epidemiological studies take shown a meaning increment in the gamble of lung, float, skin, liver and other cancers on exposure to these chemical species. Furnishings of Al, Fe, Mn, Cu, Mg and Zn on the health of living organisms are summarised in Table iii [44, 56, 67].
| Element | DWA limit | Ecological impacts of AMD |
|---|---|---|
| Al | <0.5 mg/Fifty | Prolonged exposure to aluminium has been implicated in chronic neurological disorders such equally |
| Fe | <i mg/L | Severe aesthetic effects (taste) and effects on plumbing (slimy coatings). Slight atomic number 26 overload possible in some individuals. Chronic health effects in young children and sensitive individuals in the range of 10–20 mg/L, and occasional astute effects towards the upper terminate of this range |
| Mn | <0.two mg/Fifty | Very astringent, aesthetically unacceptable staining. Domestic utilise unlikely due to adverse aesthetic effects. Some chance of manganese toxicity under unusual conditions |
| Cu | <1 mg/L | Gastrointestinal irritation, nausea and vomiting. Severe taste and staining problems. Severe poisoning with possible fatalities. Severe taste and staining issues |
| Mg | <200 mg/L | Water aesthetically unacceptable because of bitter taste users if sulphate nowadays. Increased scaling problems. Diarrhoea in almost new consumers |
| Zn | <5 mg/L | Bitter gustation; milky advent. Acute toxicity with gastrointestinal irritation, nausea and vomiting. Severe, acute toxicity with electrolyte disturbances and possible renal harm |
Table iii.
Effects of selected AMD metals on the health of living organisms.
References
- 1.
Sands P. Principles of International Ecology Law. 2nd ed. London: Cambridge; 2003 - 2.
Wong MH. Ecology Contagion: Health Risks and Ecological Restoration. Us of America: Taylor & Francis Group; 2012 - 3.
Salomons W, Forstner U, Mader P. Heavy Metals: Issues and Solutions. Berlin, Germany: Springer-Verlag; 1995 - iv.
El-Shahawi MS, Hamza A, Bashammakhb AS, Al-Saggaf WT. An overview on the accumulation, distribution, transformations, toxicity and belittling methods for the monitoring of persistent organic pollutants. Talanta. 2010; 80 :1587-1597 - 5.
Lepp NW. Upshot of heavy metal pollution on plants. Metals in the Environs, Pollution Monitoring Series, Applied Science Publishers. Department of Biology. Liverpool, Great britain: Liverpool Polytechnic; 2012; 2 - half-dozen.
Van Ael Due east, Covaci A, Blust R, Bervoets L. Persistent organic pollutants in the Scheldt estuary: Environmental distribution and bioaccumulation. Environmental International. 2012; 48 :17-27 - vii.
Elliott One thousand. Biological pollutants and biological pollution—an increasing cause for concern. Marine Pollution Bulletin. 2003; 46 :275-280 - 8.
Banfalvi G. Cellular Effects of Heavy Metals. Netherlands, London, New York: Springer; 2011 - 9.
Herawati N, Suzuki S, Hayashi Grand, Rivai IF, Koyoma H. Cadmium, copper and zinc levels in rice and soil of Nihon, Indonesia and China by soil type. Bulletin of Environmental Contamination and Toxicology. 2000; 64 :33-39 - 10.
He ZL, Yang XE, Stoffella PJ. Trace elements in agroecosystems and impacts on the environment. Journal of Trace Elements in Medicine and Biology. 2005; 19 (2–three):125-140 - 11.
Garbarino JR, Hayes HC, Roth DA, Antweiler RC, Brinton TI, Taylor HE. Contaminants in the Mississippi River: Heavy metals in the Mississippi River. Reston, Virginia: U.S. GEOLOGICAL SURVEY Circular; 1995. pp. 1133 - 12.
Brady D, Stoll Advert, Starke 50, Duncan JR. Bioaccumulation of metallic cations by Saccharomyces cerevisiae . Practical Microbiology and Biotechnology. 1994;41 :149-154 - 13.
García-Niño WR, Pedraza-Chaverrí J. Protective effect of curcumin confronting heavy metals-induced liver damage. Food and Chemical Toxicology. 2014; 69 :182-201 - 14.
Athar M, Vohora SB. Heavy Metals and Environment. New Delhi: New Age International (P) Limited; 2001 - 15.
Musilova J, Arvay J, Vollmannova A, Toth T, Tomas J. Environmental contamination by heavy metals in region with previous mining activity. Message of Environmental Contagion and Toxicology. 2016; 97 :569-575 - sixteen.
Lee G, Bigham JM, Faure G. Removal of trace metals by coprecipitation with Fe, Al and Mn from natural waters contaminated with acid mine drainage in the Ducktown Mining District, Tennessee. Applied Geochemistry. 2002; 17 (5):569-581 - 17.
Soleimani M, Amini Due north, Sadeghian B, Wang D, Fang Fifty. Heavy metals and their source identification in particulate matter (PM2.five) in Isfahan Metropolis, Iran. Journal of Environmental Sciences. 2018. In press - xviii.
Ventura LMB, Mateus VL, de Almeida ACSL, Wanderley KB, Taira FT, Saint'Pierre TD, Gioda A. Chemical composition of fine particles (PM2.5): Water-soluble organic fraction and trace metals. Air Quality, Temper and Health. 2017; ten :845-852 - 19.
Nleya Y, Simate GS, Ndlovu S. Sustainability assessment of the recovery and utilisation of acid from acid mine drainage. Journal of Cleaner Production. 2016; 113 :17-27 - 20.
Yusuf Grand, Elfghi FM, Zaidi SA, Abdullah EC, Khan MA. Applications of graphene and its derivatives as an adsorbent for heavy metallic and dye removal: A systematic and comprehensive overview. RSC Advances. 2015; v :50392-50420 - 21.
Masindi Five, Gitari MW, Tutu H. Passive Remediation of Acid Mine Drainage. LAP Lambert Bookish Publishing; 2016 - 22.
Masindi V. A novel technology for neutralizing acerbity and attenuating toxic chemic species from acrid mine drainage using cryptocrystalline magnesite tailings. Journal of H2o Process Technology. 2016; 10 :67-77 - 23.
Simate GS, Ndlovu Due south. Acrid mine drainage: Challenges and opportunities. Journal of Environmental Chemical Engineering. 2014; ii :1785-1803 - 24.
Silvas FPC, Buzzi DC, Espinosa DCR, Tenório JAS. Biosorption of AMD metals using Rhodococcus opacus . Revista Escola de Minas. 2011;64 :487-492 - 25.
Buzzi DC, Viegas LS, Rodrigues MAS, Bernardes AM, Tenório JAS. Water recovery from acrid mine drainage by electrodialysis. Minerals Engineering. 2013; 40 :82-89 - 26.
Park South-M, Shin S-Y, Yang J-S, Ji Due south-W, Baek G. Selective recovery of dissolved metals from mine drainage using electrochemical reactions. Electrochimica Acta. 2015; 181 :248-254 - 27.
Nordstrom DK, Blowes DW, Ptacek CJ. Hydrogeochemistry and microbiology of mine drainage: An update. Applied Geochemistry. 2015; 57 :3-16 - 28.
Baker BJ, Banfield JF. Microbial communities in acid mine drainage. FEMS Microbiology Ecology. 2003; 44 :139-152 - 29.
Hallberg KB. New perspectives in acid mine drainage microbiology. Hydrometallurgy. 2010; 104 :448-453 - 30.
Amos RT, Blowes DW, Bailey BL, Sego DC, Smith L, Ritchie AIM. Waste-rock hydrogeology and geochemistry. Applied Geochemistry. 2015; 57 :140-156 - 31.
Johnson DB, Hallberg KB. Acid mine drainage remediation options: A review. Science of the Total Environs. 2005; 338 :3-fourteen - 32.
Candeias C, Ávila PF, Ferreira da Silva E, Ferreira A, Salgueiro AR, Teixeira JP. Acid mine drainage from the Panasqueira mine and its influence on Zêzere river (Central Portugal), Journal of African Earth Sciences, 99, Role 2. 2014. pp. 705-712 - 33.
Akinwekomi 5, Maree JP, Zvinowanda C, Masindi Five. Synthesis of magnetite from fe-rich mine water using sodium carbonate, Journal of Environmental Chemical Technology. 2017 - 34.
Kefeni KK, Msagati TAM, Mamba BB. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. Journal of Cleaner Production. 2017; 151 :475-493 - 35.
Seo EY, Cheong YW, Yim GJ, Min KW, Geroni JN. Recovery of Fe, Al and Mn in acid coal mine drainage by sequential selective precipitation with control of pH, Catena, 148, Part 1. 2017. pp. 11-16 - 36.
Masindi V, Gitari MW, Tutu H, DeBeer M. Efficiency of ball milled south African bentonite clay for remediation of acid mine drainage. Periodical of Water Process Engineering. 2015; 8 :227-240 - 37.
Mulopo J. Continuous airplane pilot scale assessment of the alkali metal barium calcium desalination process for acid mine drainage treatment. Journal of Environmental Chemical Engineering. 2015; 3 :1295-1302 - 38.
Meschke K, Herdegen 5, Aubel T, Janneck Eastward, Repke J-U. Treatment of opencast lignite mining induced acid mine drainage (AMD) using a rotating microfiltration system. Journal of Environmental Chemic Engineering. 2015; 3 :2848-2856 - 39.
Mhamdi M, Elaloui E, Trabelsi-Ayadi M. Adsorption of zinc by a Tunisian Smectite through a filtration membrane. Industrial Crops and Products. 2013; 47 :204-211 - 40.
Gitari M, Petrik L, Etchebers O, Key D, Iwuoha E, Okujeni C. Treatment of acid mine drainage with fly ash: Removal of major contaminants and trace elements. Journal of Ecology Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering. 2006; 41 :1729-1747 - 41.
Van der Linde Thousand, Feris L. Compendium of South African Environmental Legislation. Pretoria, South Africa: Pretoria University Law Press; 2010 - 42.
Madzivire G, Gitari WM, Vadapalli VRK, Ojumu TV, Petrik LF. Fate of sulphate removed during the treatment of circumneutral mine h2o and acid mine drainage with coal fly ash: Modelling and experimental arroyo. Minerals Engineering. 2011; 24 :1467-1477 - 43.
Africa S, Van der Linde M, Feris Fifty. Compendium of Southward African Environmental Legislation. Pretoria University Law Press; 2010 - 44.
Tutu H, McCarthy TS, Cukrowska E. The chemical characteristics of acrid mine drainage with particular reference to sources, distribution and remediation: The Witwatersrand Basin, South Africa as a case study. Applied Geochemistry. 2008; 23 :3666-3684 - 45.
Madzivire 1000, Petrik LF, Gitari WM, Ojumu TV, Balfour M. Application of coal fly ash to circumneutral mine waters for the removal of sulphates as gypsum and ettringite. Minerals Engineering. 2010; 23 :252-257 - 46.
Biswas AK, Tortajada C, Izquierdo R. Water Quality Management: Nowadays Situations, Challenges and Future Perspectives. New York, U.s.a.: Taylor & Francis; 2014 - 47.
Madzivire Thousand, Maleka P, Lindsay R, Petrik LF. Radioactive decay of mine water from a gold mine in South Africa. WIT Transactions on Ecology and the Environment. 2013; 178 :147-158 - 48.
Madzivire Thou, Maleka PP, Vadapalli VRK, Gitari WM, Lindsay R, Petrik LF. Fate of the naturally occurring radioactive materials during handling of acid mine drainage with coal fly ash and aluminium hydroxide. Journal of Ecology Direction. 2014; 133 :12-17 - 49.
Masindi V, Gitari MW, Tutu H, De Beer M. Awarding of magnesite–bentonite clay composite every bit an alternative technology for removal of arsenic from industrial effluents. Toxicological & Ecology Chemical science. 2014:1-17 - 50.
Gitari WM, Petrik LF, Etchebers O, Primal DL, Iwuoha E, Okujeni C. Passive neutralisation of acid mine drainage by fly ash and its derivatives: A cavalcade leaching report. Fuel. 2008; 87 :1637-1650 - 51.
Masindi V, Gitari MW, Tutu H, De Beer M. Application of magnesite–bentonite clay composite as an alternative technology for removal of arsenic from industrial effluents. Toxicological & Environmental Chemistry. 2014; 96 :1435-1451 - 52.
Torres E, Ayora C, Jiménez-Arias JL, García-Robledo East, Papaspyrou S, Corzo A. Benthic metallic fluxes and sediment diagenesis in a water reservoir affected past acid mine drainage: A laboratory experiment and reactive send modeling. Geochimica et Cosmochimica Acta. 2014; 139 :344-361 - 53.
Šucha 5, Dubiková M, Cambier P, Elsass F, Pernes M. Issue of acrid mine drainage on the mineralogy of a dystric cambisol. Geoderma. 2002; 110 :151-167 - 54.
Peretyazhko T, Zachara JM, Boily JF, Xia Y, Gassman PL, Arey BW, Burgos WD. Mineralogical transformations controlling acid mine drainage chemistry. Chemical Geology. 2009; 262 :169-178 - 55.
Netto E, Madeira RA, Silveira FZ, Fiori MA, Angioleto E, Pich CT, Geremias R. Evaluation of the toxic and genotoxic potential of acrid mine drainage using physicochemical parameters and bioassays. Ecology Toxicology and Pharmacology. 2013; 35 :511-516 - 56.
Mohapatra BR, Douglas Gould W, Dinardo O, Koren DW. Tracking the prokaryotic diversity in acid mine drainage-contaminated environments: A review of molecular methods. Minerals Engineering. 2011; 24 :709-718 - 57.
Martins Thou, Santos ES, Faleiro ML, Chaves S, Tenreiro R, Barros RJ, Barreiros A, Costa MC. Performance and bacterial customs shifts during bioremediation of acrid mine drainage from two Portuguese mines. International Biodeterioration & Biodegradation. 2011; 65 :972-981 - 58.
Levings CD, Varela DE, Mehlenbacher NM, Barry KL, Piercey GE, Guo M, Harrison PJ. Effect of an acid mine drainage effluent on phytoplankton biomass and primary production at Britannia Beach, Howe Sound, British Columbia. Marine Pollution Bulletin. 2005; 50 :1585-1594 - 59.
Sun M, Ru Ten-R, Zhai Fifty-F. In-situ fabrication of supported iron oxides from constructed acid mine drainage: Loftier catalytic activities and expert stabilities towards electro-Fenton reaction. Applied Catalysis B: Ecology. 2015; 165 :103-110 - 60.
Strosnider WH, Nairn RW. Effective passive treatment of high-strength acid mine drainage and raw municipal wastewater in Potosí, Bolivia using simple mutual incubations and limestone. Journal of Geochemical Exploration. 2010; 105 :34-42 - 61.
Mitsch WJ, Wise KM. Water quality, fate of metals, and predictive model validation of a synthetic wetland treating acrid mine drainage. Water Research. 1998; 32 :1888-1900 - 62.
Mapanda F, Nyamadzawo G, Nyamangara J, Wuta M. Effects of discharging acrid-mine drainage into evaporation ponds lined with dirt on chemical quality of the surrounding soil and water. Physics and Chemistry of the Globe, Parts A/B/C. 2007; 32 :1366-1375 - 63.
Mahmoud KK, Leduc LG, Ferroni GD. Detection of Acidithiobacillus ferrooxidans in acid mine drainage environments using fluorescent in situ hybridization (FISH). Journal of Microbiological Methods. 2005; 61 :33-45 - 64.
Grey NF, Delaney E. Measuring customs response of bentic macroinvertebrates in an erosional river impacted past acrid mine drainage by use of a simple model. Ecological Indicators. 2010; 10 :668-675 - 65.
Gray JB, Vis ML. Reference diatom assemblage response to restoration of an acrid mine drainage stream. Ecological Indicators. 2013; 29 :234-245 - 66.
Skoczyńska-Gajda Due south, Labus Thousand. Acid mine drainage within the abased lignite mining area-Muskau Arch. Biuletyn Państwowego Instytutu Geologicznego. 2011:643-650 - 67.
Rubin H, Rubin K, Siodlak A, Skuza P. Assessment of contamination of the bottom sediments of the Stola River with selected metals and metalloids within the urban-industrial surface area of Tarnowskie Góry. Biuletyn Państwowego Instytutu Geologicznego. 2011:615-624 - 68.
Jooste S, Thirion C. An ecological risk assessment for a Due south African acid mine drainage. Water Science and Technology. 1999; 39 :297-303
Submitted: October 6th, 2017 Reviewed: March 1st, 2018 Published: June 27th, 2018
© 2018 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License, which permits unrestricted utilize, distribution, and reproduction in any medium, provided the original work is properly cited.
garrisonandly1960.blogspot.com
Source: https://www.intechopen.com/chapters/60680
0 Response to "many of the heavy metals are what to living things"
Post a Comment